Clinical Trials
Qscan Group is invested in making a difference in the medical field, and to translate innovation into clinical practice. Imaging for clinical trials is one way in which the Qscan Group contributes to such innovation.
Qscan Group takes a human-centric approach to clinical trials and operates with compliance and security at our core. We are flexible, agile, and keen to improve diagnostic imaging and reporting with the help of artificial intelligence (A.I.) software.
Our participation in clinical trials is underpinned by our state-of-the-art imaging facilities and our treatment teams, which consist of doctors, nurses, and technical staff.
We ensure appropriate and meaningful engagement with our stakeholders occurs at every stage of the trial, from initial design to dissemination. Our quality review processes also ensure all participants receive the highest standard of attention and care, which matches the high quality service we provide to all patients that visit our clinics.
Our Focus
Qscan Group is focused on assisting in clinical trials for cancer treatments and interventional radiology.
We are passionate about improving access to clinical trials for all participants and forging new partnerships and collaborations.

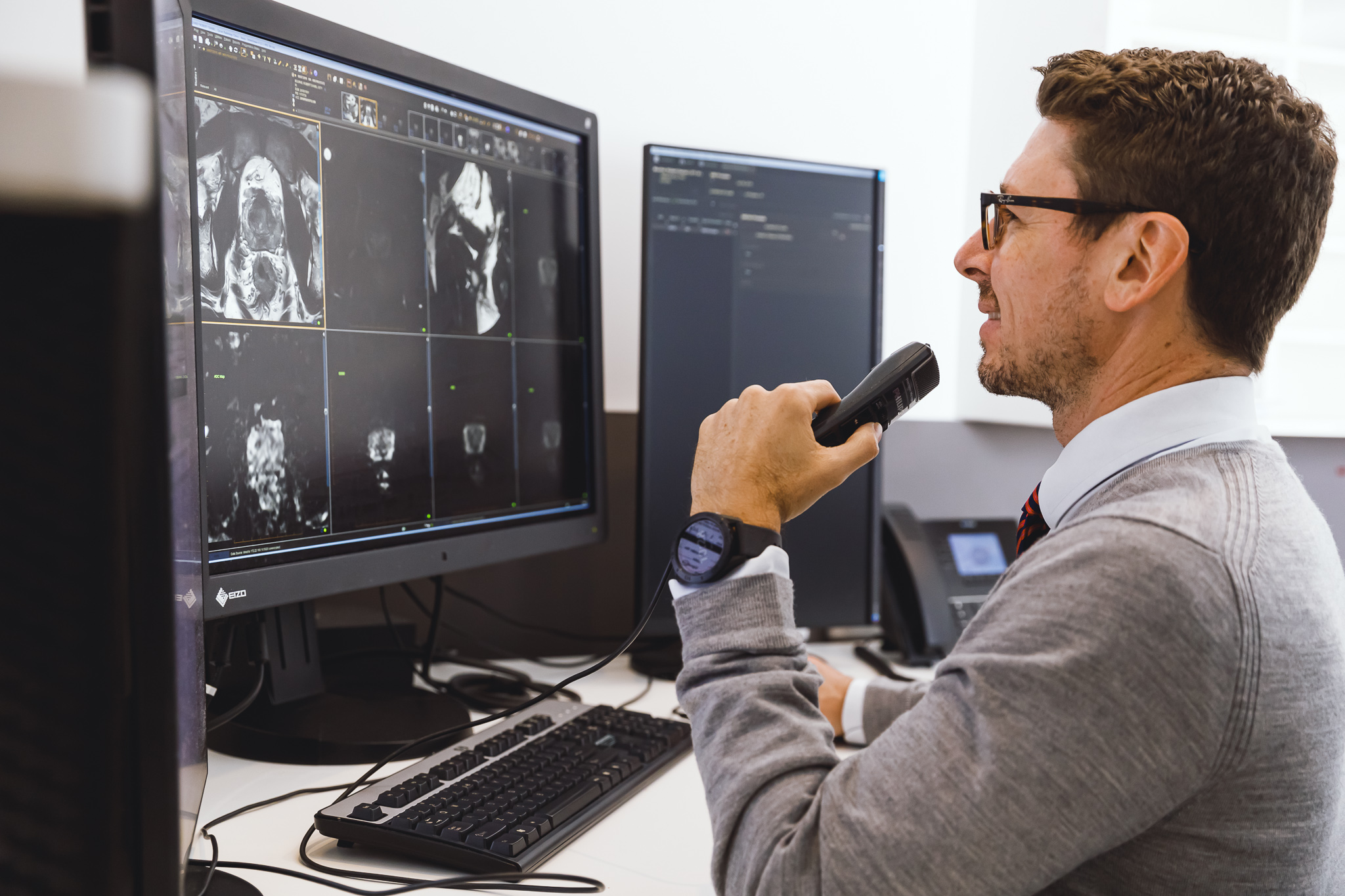
Reporting Criteria
- a reliable group of expert radiologists who partner with your research team for the long-term
- doctors are accessible at all times to ensure consistency, reliability, and accuracy
- research team is able to liaise directly with doctors for clarification and education
- research team is available for discussions with trial sponsors to ensure the best outcomes for your trial projects
- reporting will align with study protocol and imaging requirements provided (i.e., RECIST V1.1)
FAQs
For all new clinical trial enquiries, please email clinicaltrials@qscan.com.au the following completed documentation:
1. New Clinical Research Enquiry Form
2. Study Protocols
3. Imaging Requirements (if available)
Note: Items 1 and 2 are a mandatory to enable our team to provide a quote on your upcoming study.
It will take about 20 business days from receipt of your completed enquiry form and appropriate study protocols.
The following table provides approximate time frames for each stage of the process.
| Step | Turnaround time |
| Client submits new request | Dependent on client |
| Quote prepared | 5 business days |
| Quote accepted by client | Dependent on client |
| Contract prepared | 5 business days |
| Contract signed by client | Dependent on client |
| Contract executed | 10 business days |
| Set up clinical trial activities | Occurs in parallel to execution of contract |
| Trial active, patients seen | Dependent on trial duration |
| Clinical trial complete | |
Please note this is a guide only, more complex requests and/or incomplete/changing requests can take longer to process.
The study start-up fee covers the administration required to establish and manage a clinical trial.
Clinical trial scans differ from standard patient care services, which means additional resources must be allocated to understand and prepare our teams
Please email your updated study protocols with your study protocol name in the subject of the email to clinicaltrials@qscan.com.au, highlighting any changes for our immediate attention.
Once the changes have been reviewed, we will let you know if our fees have changed. If they have changed, we will amend your quote as appropriate.
Unfortunately, Qscan Group cannot submit any bill to Medicare or any private health insurer on behalf of clinical trial patients or sponsors. This is not permitted under section 20A of the Health Insurance Act.
If a patient is being seen under an approved and active clinical trial, Qscan Group will invoice the sponsor or client of the clinical trial in full.
If the clinical trial is no longer active, the patient can be referred to Qscan Group through normal channels, where bulk billing will apply.
This differs from standard patient services, which patients can claim against Medicare and private health insurance providers where allowed. Under section 20A of the Health Insurance Act, Medicare rebates cannot be claimed for clinical trial services.
Requests for specific clinics and/or radiologists can be noted in the ‘New Clinical Trial Request Form’. Our team will let you know if your request can be accommodated – this will be dependent on staff and/or machine availability.
Please email clinicaltrials@qscan.com.au if you have any other enquiries – in this email, please let us know if you would prefer a response by phone or email.
Our team will aim to respond to your enquiry within 2 business days.
Contact Us
For all clinical trial enquiries, please contact us
